|
Eric J. Werner
|
Research Interests
Research in our lab focuses on the synthesis of new molecules capable of binding specific lanthanide or actinide metal ions for a variety of biomedical and environmental applications. Novel f-element chelators are being prepared for applications ranging from luminescent sensors to extractants for improved lanthanide/actinide separation technologies. For example, complexes of select lanthanide ions (e.g. Eu3+ and Tb3+) are currently being evaluated with regard to their luminescent properties to enable imaging or sensing of biologically relevant targets. The relatively long luminescence decay lifetimes, sharp emission bands and large Stokes shifts of the sensitized emission make these ions ideal as probes under biological conditions. In addition to these sensor studies, current collaborative extensions of the work involve the encapsulation of the small molecule chelates within silica-based nanoparticles. The combination of the unique photophysical properties of the lanthanides with the well-known chemistry and diverse applicability of silica nanoparticles may yield highly emissive materials for use in a variety of areas including biomedical imaging.
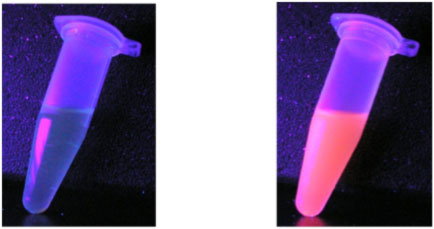
Solution of a Eu complex excited by UV light (left) and the same complex encapsulated by silica nanoparticles illuminated under identical conditions (right).
With support from the National Science Foundation (NSF grant award no. 2102381), current directions within the lab have also focused on the development of selective extractants of rare earth elements and, in some cases, actinides. Due to the large volumes of nuclear waste materials already present and the need for alternative energy sources which may include nuclear, improved methods for waste remediation due to safety and environmental concerns are desirable. The increasing use of lanthanide metals across many areas of modern technology and alternative energy applications also creates a need for acquiring these valuable metals more efficiently. The development of improved f-element extraction agents would enable metal mining with less environmental impact as well as allow for rare earth element recycling from electronic waste. Fundamental to this challenge is the design of molecules with enhanced metal extraction capability, inspiring further chelator development and f-element coordination chemistry research in our group.

Undergraduate student Tavya Benjamin synthesizing a new macrocyclic Eu3+ complex for luminescent sensor applications.